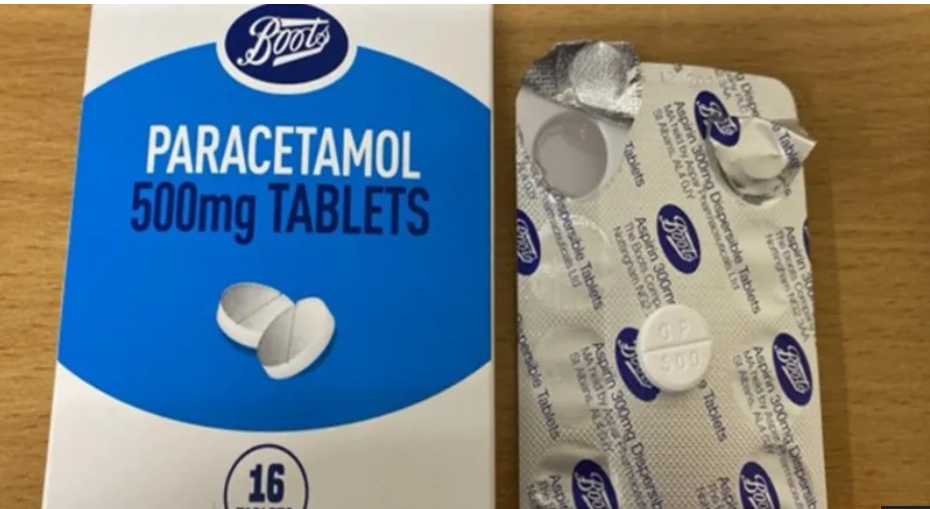
Boots has issued a recall for packs of 500mg paracetamol tablets following a labelling error that incorrectly identifies them as aspirin.
More than 110,000 affected packs, marked with batch number 241005 and an expiry date of 12/2029, are being recalled. Customers are advised to stop using the product and return it to any Boots store for a full refund, even without a receipt.
Labelling Mix-Up
While the outer packaging correctly states “Paracetamol 500mg Tablets,” the inner foil blister pack is mislabelled as “Aspirin 300mg Dispersible Tablets.”
Boots and supplier Aspar Pharmaceuticals Limited have launched an investigation into the error. The company warns that using the medication despite being aware of the mislabelling could lead to incorrect dosing.
MHRA Warning
Dr. Stephanie Millican of the Medicines and Healthcare Products Regulatory Agency (MHRA) has urged consumers to check their packs and return affected batches immediately.
“If you are unsure which pack you have purchased or have experienced any side effects, seek advice from a healthcare professional,” she stated. Suspected adverse reactions should be reported through the MHRA’s Yellow Card scheme.
Paracetamol vs. Aspirin
Paracetamol is commonly used for mild to moderate pain, such as headaches, toothaches, and stomach aches. Aspirin, on the other hand, is often preferred for period pain or migraines and works by reducing swelling and high temperature.
The recall highlights the importance of accurate labelling to prevent potential health risks. Customers are advised to return any affected packs as soon as possible and consult a pharmacist if they have concerns
